The Dynamic European Forest
Author: Frans W.M. Vera*
|
To cite this article:
|
To cite this article: Frans W.M. Vera (2002) THE DYNAMIC EUROPEAN FOREST, Arboricultural Journal, 26:3, 179-211, DOI: 10.1080/03071375.2002.9747335
|
|
To link to this article:
|
To link to this article: https://doi.org/10.1080/03071375.2002.9747335
|
|
Published online:
|
Published online: 27 Mar 2012
|
|
Publication:
|
Arboricultural Journal 2002, Vol. 26, pp. 179-211
|
|
Copyright:
|
© AB Academic Publishers 2002 Printed in Great Britain
|
This paper was originally presented at the 25th National Arboriculture Conference, University of Lancashire, September 2001.
The paper is based on the book: F.W.M. Vera (2000) Grazing Ecology and Forest History. CABI Publishing, Wallingford. The number of references in this paper has been restricted. The full number can be found in the book. The book can be looked into on the following Internet address: http://www.cabi.org/Bookshop/ReadingRoom/0851994423.asp
*Directic Kabinet, Ministry of Agriculture, Nature Management and Fisheries, Postbus 20401, 2500 EK, 's-Gravenhage, The Netherlands. E-mail: f.w.m.vera@ision.nl
Summary
It is a generally accepted theory that, had there been no human intervention, closed-canopy forest would predominate in Central and Western Europe in places where trees can grow. Apparent evidence for the past presence of closed-canopy forest comes from several sources: abandoned pastures reverting to forest; palynological studies; and linguistic analysis. However, VERA (2000) shows that these concepts are erroneous in several key respects. Oak (Quercus species) and hazel (Corylus avellana) are light-requiring species and their presence in palynological studies, together with the presence of many bones from cattle and horses, actually indicates the presence of open ground. Linguistic analysis also shows that the terms ‘forest’ and ‘wood’ did not imply simply a solid cover of trees, but rather the uncultivated ground – that is not ploughed or mowed – and with or without trees and shrubs – ‘outside the settlement’. These new interpretations are combined with evidence for the role of blackthorn (Prunus spinosa) together with the grazing of large ungulates like (wild) cattle and (wild) horse as an aid to scrub (and high-forest) regeneration. It is concluded that Europe has not been totally covered by closed-canopy forest in places where trees can grow. In places where wild cattle and wild horses could live together with other large ungulates it was originally made up of dynamic pasture woodland.
Keywords: pasture woodland • forest history • historical ecology • linguistic analysis • palynology
It is a generally accepted theory that in the natural state, if there had been no human intervention, the lowlands of Central and Western Europe, with their temperate climate would have been covered with a closed-canopy forest in places where trees can grow. Regeneration of the trees forming these forests would have taken place in gaps in the canopy according to the gap phase model of WATT (1947) or the cyclical model of LEIBUNDGUT (1959, 1978). In these models gaps are created when one or several trees die or are windblown.
The theory of the closed-canopy forest is to a significant extent based on the observation that abandoned fields and pastures where people withdrew livestock change spontaneously into a closed-canopy forest. Underlying this theory is the premise that the original fauna of large herbivores did not have a decisive influence on the forest concerning its species composition and regeneration. Wood-pastures like the New Forest (see Figure 1) are considered to be degraded closed-canopy forests. This is based on the knowledge that livestock and wild ungulates, if present in certain densities, are able to prevent the regeneration of the trees in the forest by trampling and eating the young trees: The forest becomes more and more open. It is said to degrade into a park-like landscape, and finally into grassland or heathland as a result of retrogressive succession (see MOSS, 1910, p. 36; WATT, 1919; TANSLEY, 1953, pp. 129-130; ELLENBERG, 1986, p. 43). Fenced parts of such forests show prolific regeneration of trees. They prove the potential threat of large ungulates for the regeneration of forests (see PETERKEN and TUBBS, 1965; PUTMAN et al., 1989; MOUNTFORD et al., 1999). This would show that the indigenous large ungulates were present in the primaeval vegetation in very low densities (TANSLEY, 1953, p. 143,487). So, a forest degraded by grazing of cattle will revert to its natural situation - a closed-canopy forest - once grazing is stopped. This happened in many forest reserves in the lowlands of central and western Europe. This is based on succession theory, which states that under certain climatological, soil and hydrological conditions bare ground develops into a plant community of a particular type. This is dominated by the largest and tallest plants, which are able to thrive in the prevailing climatological conditions because they are the strongest in the competition for light (CLEMENTS, 1916, pp. 3, 63, 80, 99, 125; TILMAN, 1985). If these are trees - as in the lowlands of Europe - the final stage is a forest.

FIGURE 1. The wood-pasture New Forest in England. It consists of open areas and groves or boscages that are all grazed by large ungulates. The photo shows the inside of a grove, a so-called ornamental wood. Photograph F.W.M. Vera.
Another important argument comes from the interpretation of the regulations for the grazing of livestock in what are supposed to be the last remaining wildernesses in the lowlands of Central and Western Europe from the Middle Ages onwards. It is argued that pasturing livestock was temporarily forbidden in order to make it possible for saplings of trees to grow up in the forest.
The results of pollen studies are said to confirm the theory that the primaeval vegetation was a closed-canopy forest. The most used argument is that up to 90 per cent of the pollen originates from broad-leaved deciduous forest trees and the shrub hazel (Corylus avellana). According to the pollen the forest would have consisted of species like oak (Quercus robur and Q. petraea), lime (Tilia cordata and T. platyphyllos), elm (Ulmus spp.), ash (Fraxinus excelsior), beech (Fagus sylvatica) and hornbeam (Carpinus betulus) with a shrub layer containing hazel (see VON POST, 1916; FIRBAS, 1934; 1935; 1949, p. 1; GODWIN, 1934a,b; IVERSEN, 1960; VERA, 2000, p. 3). The palaeoecologists base their interpretation of prehistoric pollen on the theories about succession as mentioned above (see GODWIN, 1934b; IVERSEN, 1941, pp. 21,36,44,47; 1960, pp.6,26-27; VERA, 2000,pp. 13-60, 70-75).
Bones that are found show that at that times a fauna of large ungulates existed. It consisted of aurochs (Bos primigenius) and tarpan (Equus przewalski gmelini), the wild ancestor of cattle and the domestic horse. These two species roamed and grazed the primaeval vegetation. They did so in combination with other wild indigenous ungulates, namely European bison (Bison bonasus), red deer (Cervus elaphus), elk (Alces alces), roe deer (Capreolus capreolus) and wild boar (Sus scrofa) (DEGERBOL, 1964; DEGERBOL and IVERSEN, 1945; AARIS-SORENSEN, 1980; AARIS-SORENSEN et al., 1990; AUGUSTE and PATOU-MATHIS, 1994). Palaeoecologists assume that the indigenous wild ungulates have had no effect on the regeneration of the primaeval forest, because otherwise the primaeval vegetation could not have been a closed-canopy forest. Therefore, the animals should have occurred naturally in very low numbers (see IVERSEN, 1960, p. 26; 1973, pp. 72-73).
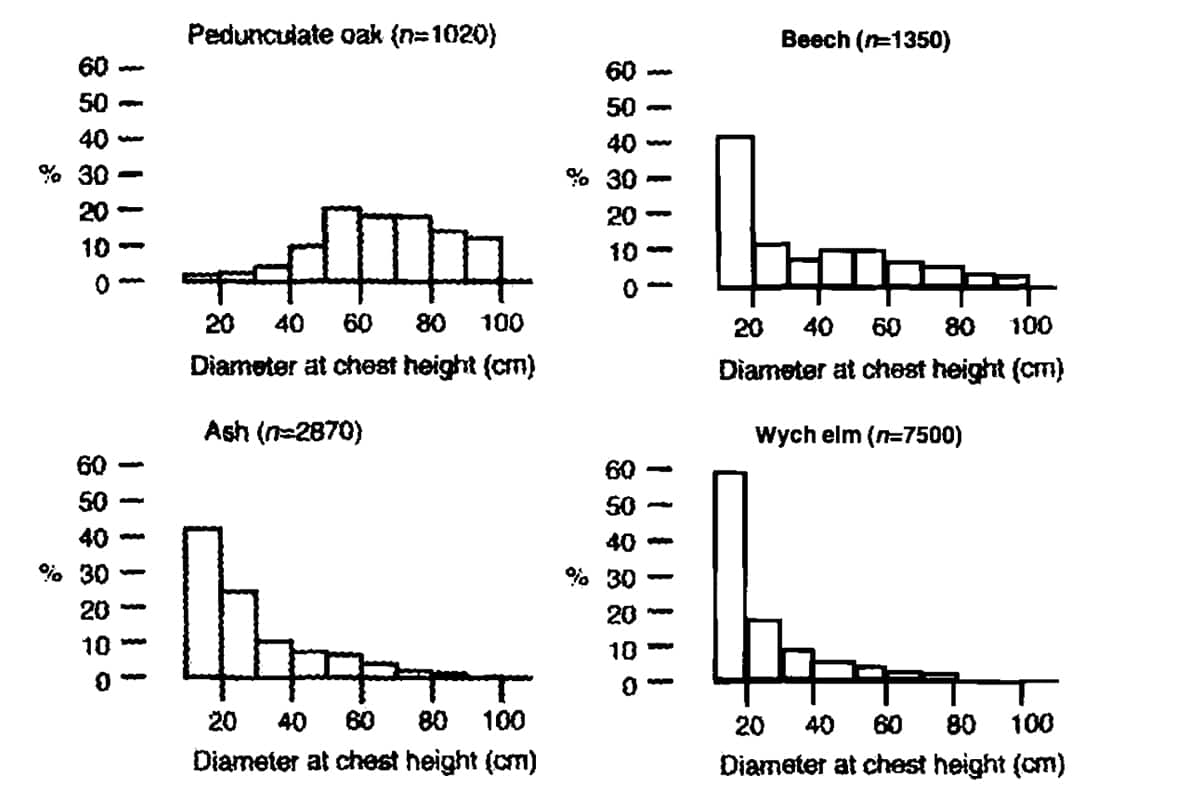
FIGURE 2. The percentage distribution per species of tree divided in diameter categories of the main tree species in Dalby Soderskog, Sweden. Only trees with a trunk diameter of >10 cm at chest height are included (redrawn from MALMER et al., 1978, p. 20).
The problem
The characteristics of the tree species of which pollen have been found are derived from the theory of the closed-canopy forest. It means that a tree species like oak is considered to be a forest tree. It should therefore be able to regenerate within gaps in the canopy of the forest as well as other tree species. The same should apply for the other tree species as well as for shrubs like hazel. It follows that all species of trees and shrubs which pollen studies have shown to be present in the undisturbed prehistoric forest will survive in spontaneously developing forests, so-called 'forest reserves', and regenerate spontaneously when gaps in the canopy are formed.
However, this does not apply in these forest reserves, to pedunculate oak (Quercus robur), sessile oak (Q. petraea) and hazel (Corylus avellana). The diameter distribution of oak is ‘clock shaped’, indicating that it is a population that is dying out. This is contrary to species like beech (Fagus sylvatica), ash (Fraxinus excelsior), elm (Ulmus glabra and U. laevis), lime (Tilia platyphyllos and T. cordata) and hornbeam (Carpinus betulus) (see inter alia LODL et al., 1977; MALMER et al., 1978; KOOP, 1981; LEMEE, 1985; 1987; PETERKEN and JONES, 1987; EMBORG et al., 1996; VERA, 2000, pp. 189-286).
They display an inverse J-curve. This indicates a successfully regenerating population (see Figure 2). The conclusion drawn from these figures by investigators is that pedunculate and sessile oak and hazel are disappearing from those forest reserves (see inter alia LODL et al., 1977; JAHN and RABEN, 1982; HYTTEBORN, 1986; LEMEE, 1987; EMBORG et al., 1996; and VERA 2000, pp. 191-286). These light-demanding species are ousted by shade-tolerant tree species like lime, elm, ash, sycamore, beech, and hornbeam (see KOOP, 1981; LEMEE, 1985; 1987; MALMERet al., 1978; LODL et al., 1977; EMBORG et al., 1996; VERA, 2000, pp. 189-286).
This is not in agreement with pollen diagrams from the primaeval vegetation. They show that both oak and hazel were very well represented in this vegetation for many thousands of years together with the shade-tolerant species. Therefore, these species must have been able to regenerate successfully in the presence of shade-tolerant species (see for instance FIRBAS, 1934; 1935; 1949; IVERSEN, 1941; 1960; 1973; HUNTLEY and BIRKS, 1983; PEGLAR, 1993; HANNON et al., 2000; VERA, 2000, p. 7). So, it can be questioned whether the theory of the primaeval vegetation being a closed-canopy forest is valid.

FIGURE 3. Tree species like birch, hazel, hornbeam and lime emerging from mantle and fringe vegetation consisting of blackthorn, hawthorn and hazel, western Jura, France. Notice especially the young oak trees on the foreground, surrounded by blackthorn (photograph F.W.M. Vera).
At the same time, oak and hazel do regenerate very well in the presence of shade-tolerant tree species in so-called wood-pastures (see inter alia FORBES, 1902; WATT, 1919; 1924; TANSLEY, 1953, pp. 130-133; PETERKEN and TUBBS, 1965; HART, 1966, pp. 180-181, 186,209,225,310; FLOWER, 1977,pp.28,32; 1980; BURRICHTER et al., 1980; RACKHA , 1980, pp. 173, 293; ELLENBERG, 1988, pp. 20-21, 33-34; RODWELL, 1991, pp. 333-351; PIETZARKA and ROLOFF, 1993). These are park-like landscapes where domesticated large herbivores like cattle and domestic horse graze the vegetation in combination with wild ungulates like deer. Such a landscape consists of grassland, scrub, solitary trees and groves. Shrubs mark the transition of the grassland to the grove and form the so-called mantle and fringe vegetation on the outline of the groves (see Figure 3). So, the question arises whether these wood-pastures could be considered as modem analogues of the primaeval vegetation. If that is the case, livestock in combination with the wild ungulates must have acted upon the vegetation as an analogue of the wild fauna. To be more specific, the effect of cattle and horses on the vegetation in wood-pastures could be considered as a modem analogue of the effect of their wild ancestors, aurochs (Bos primigenius) and tarpan (Equus przewalski gmelini). Those species roamed and grazed the primaeval vegetation in combination with the other wild indigenous ungulates European bison (Bison bonasus), red deer (Cervus elaphus), elk (Alces alces), and roe deer (Capreolus capreolus) (DEGERBOL, 1964; DEGERBOL and IVERSEN, 1945; AARIS-SORENSEN, 1980; AARIS-SORENSEN, et al., 1990; AUGUSTE and PATOU MATHIS, 1994).
The arguments used in favour of the theory of the closed-canopy forest
The first and main argument in favour of the theory of the closed-canopy forest comes from the interpretation of observation on the spontaneous development of the vegetation on abandoned arable fields and meadows abandoned by cattle after people withdrew them (see COTTA, 1865, pp. v; FORBES, 1902; TANSLEY, 1911, pp. 7-8; 1953, pp. 293-294; CLEMENTS, 1916, pp. 145, 151, 155; WATT, 1919; 1947). It is stated that people actually disturbed nature by introducing agriculture. People did so by creating fields for crops and introducing livestock into the primaeval vegetation. If agricultural intervention were ended, of course, nature would begin again, starting with a spontaneous development of the vegetation and ending with so-called 'climax' vegetation. On places where trees can grow this climax vegetation would be a closed-canopy forest. This is based on the succession theory, which states that under certain climatological, soil and hydrological conditions bare ground develops into a plant community of a particular type. This is dominated by the largest and tallest plants, which are able to thrive in the prevailing climatological conditions because they are the strongest in the competition for light (CLEMENTS, 1916, pp. 3, 63, 80, 99, 125; TANSLEY, 1935; TILMAN, 1985).
If these are trees - like in the lowlands of Europe - the final stage is a forest. If the climax forest disappeared as a result of some form of human intervention, like the introduction of livestock, the climax will spontaneously develop again. This happens by means of secondary succession, if people withdraw livestock and the intervention effectively ceases (CLEMENTS, 1916, pp. 60, 63, 107, 176; TANSLEY, 1953, pp. 130, 293-295, 487). In the lowlands of Europe the climax vegetation is supposed to be a broad-leaved closed-canopy forest. It would have consisted of species like oak (Quercus robur and Q. petraea), lime (Tilia cordata and T. platyphyllos), elm (Ulmus spp.), ash (Fraxinus excelsior), beech (Fagus sylvatica) and hornbeam (Carpinus betulus) with a shrub layer containing hazel (see VERA, 2000, p. 3).
The second main argument - also based on the former - is the interpretation of historical sources. It concerns the regulations from the beginning of the Middle Ages onwards for cattle-grazing in the last wildernesses in the lowlands of Europe. Foresters use this argument to show how people in the Middle Ages took measurements in order to preserve the last original forests. People would have done so because of their dependence of forest in those times for firewood and timber (see HAUSRATH, 1898, p. 101; 1982, pp. 39, 206- 209; BUHLER, 1922, pp. 300-301, 339, 610; MEYER, 1931, pp. 345, 386; DENGLER, 1935, pp. 75-84). The interpretation of pollen diagrams by palynologists was to a large extent based on these two arguments (see VON POST, 1916; BERSTCH, 1929; 1932; 1949, p. 4; TSCHADEK, 1933; FIRBAS, 1934; 1935; 1949, p. 1; GODWIN, 1934a,b; IVERSEN, 1941, p. 21, 36, 44, 47; 1960, pp.6-7, 26-27).
Regulating the use of the wilderness
From the 7th century onwards charters are known in which Merovingian and Frankish kings declared the uncultivated wilderness as ‘forestis nostra’, or ‘forestis’. The theory, which is most widely supported about the origin of the concept ‘forestis’, is that it is derived from the Latin ‘foris’ or ‘foras’. These mean ‘outside’, ‘outside it’, and ‘outside the settlement’ (see KASPERS, 1957, p. 24; HESMER, 1958, p. 408; BUIS, 1985, p. 26 et seq.; MULLER and RENKEMA, 1995, p. 363; see Figure 4). So it concerned the area outside the cultivated, the settlement, the fields of arable land and the hay-fields. According to the legal order in those times this area had no clear owner. It was the so-called ‘bona vacantis’ that, based on Roman Law, the Codex Justinianus X, JO, belonged to the ‘government’, or the king (DE MONTE VERLOREN and SPRUIT, 1982, p. 123).
‘Forestis’ was a legal concept. It described or confirmed the royal rights concerning the ownership and the right to use (KASPERS, 1957, pp. 23-25; BUIS, 1985, pp. 26; MANTEL, 1990, pp. 63-67). It applied to the wilderness in general, and to trees, shrubs, wild animals, water and fish in particular (KASPERS, 1957, pp. 24-30; HESMER, 1958, p. 408;MANTEL, 1980, p. 1005; BUIS, 1985, pp. 25-26, 223 et seq.). It meant that in a ‘forestis’, every individual tree, as well as every wild animal, belonged to the king, and that only the king had the right to make use of these. Without his express consent, others were not permitted to graze their livestock, to cut or to collect firewood, to fell trees, to create fields for crops or to hunt animals in the 'forestis' (KASPERS, 1957, pp. 23-26, 39- 40).

FIGURE 4. Settlement in the 16th century. From the 7th century the area outside the fence surrounding the settlement where deer and hare are was declared by the king as ‘forestes nostra’. In the common language this area was called in the Saxon language wald, weld or wold and in the Anglo-Saxon weald. In the Dutch language it was called woud.
When the wilderness was declared to be a ‘forestis’ it was subject to the ‘ius forestis’. In German this was called ‘Waldrecht’ and in Dutch 'woudrecht' (translated in English ‘forest law’, not to be confused with the Forest Law, later on in the 11th century introduced in Britain by William the Conqueror from the continent to England when he became king there). The king passed the administration and management of the ‘forestis’ to officials he appointed, so-called ‘forestarii’. They regulated the use and dealt with the infringements of the regulations. They did so in a legal forum, a court, in accordance with the ‘ius forestis’, the 'iura forestarorium' or the ‘ius nemoris’ (KASPERS, 1957, p. 32-39, 50; BUIS, 1985, p. 223, 225; BUIS, 1993, p. 41). According to these regulations, the king allowed local communities to use the ‘forestis’ as common land for pasturing cattle and pigs and for collecting leaf fodder for livestock, for firewood and for getting timber.
The oldest rules concerning the management of the ‘forestis’ date from the 6th century. Up to the 13th century they were concerned only with the payments by commoners to the lord for the pannage of pigs in the 'forestis' and the protection of so-called ‘fruitful’ trees. The fruitful trees were oak, wild apple (Malus sylvestris), wild pear (Pyrus pyraster) and wild cherry (Prunus avium) (see KASPERS, 1957, pp. 166; RACKHAM, 1975, pp. 27; TUBBS, 1964, pp. 96; HART, 1966, pp. 95; FLOWER, 1977, pp. 26; HAUSRATH, 1982, pp. 28-29; BUIS, 1985, pp. 304-305). These trees provided fruits (acorns, pears, apples and cherries), called mast, for pasturing pigs that were fattened with them. The most important mast consisted of acorns. In Anglo-Saxon acorn meant 'recer' (in Saxon 'Acker'). Pigs were fattened on the 'recer' or 'Acker'. In German this is called 'ackern' and in Dutch 'ackeren'. In English it is called pannaging, after the French 'pannage'. The 'Acker' was also the place where the acorns were found and the pigs were driven to. So, the 'Acker', was named after what it provided, namely: 'Acker' (acorns, mast, pannage). The 'Acker' was therefore also the place in the wilderness where light-demanding oaks and other fruit bearing (all light-demanding) trees were standing (see VERA, 2000, pp. 123-126).
During the Middle Ages, the cutting of foliage was increasingly regulated in the sense of restricted, because of the damage which was caused to the trees. It prevented, for example, the trees from flowering and therefore producing fruit (ENDRES, 1888, p. 54; MANTEL, 1980, pp. 103-104, 696, 934, 941, 969, 977, 984, 992; VERA, 2000, p. 103, 111, 123, 129-130). Eventually it became entirely prohibited.
The foliage was called in German and Dutch 'weide', from the Frankish concept 'weide', which meant 'food' 'collecting food', 'looking for food' and 'the place where food was found' (DE VRIES, 1970, p. 249; VAN VEEN and VAN DER SUS, 1991, p. 817). Animals who collect food 'weiden' (pasture, or are pasturing). So in the Middle Ages a tree as well as grassland was a pasture, a place with food. A tree was not only the pasture for cattle, but also for the pigs, namely the acorns, the 'Acker'. Therefore an oak was a pasture. Because the concept pasture in the Middle concerned trees as well as grasses and herbs, no distinction was made between grassland and trees as is done nowadays by speaking of wood and pasture. In the Middle Ages it made no sense doing so, because pasture was not a concept clifferent from woods. Trees and grasses was all pasture for animals.
There are strong indications that grasslands were part of 'Wald' and 'forestis'. Animals were driven on the 'Blume' (on the flowers) in the 'Wald' or 'forestis'. This meant they were driven in the summer time in the 'Wald'. During the summer open grasslands are flowering. Therefore open grasslands were an integral part of the 'forestis' and the 'Wald'. Sometimes a 'Wald' was described as being extremely suitable for pasturing livestock (see VERA, 2000, pp. 103-115).
There are more indications that open treeless areas were part of both the 'forestis' and the 'Wald'. There were no regulations whatsoever to indicate that trees were removed in order to create pasture for livestock in the modern sense of the word. On the contrary, the rule was that it was strictly forbidden to fell trees. Only for making fields to raise crops was permission granted to grub up trees. Besides this purpose, trees were only allowed to be felled for building houses. This affected especially oak, but then only as much as people needed was permitted and only with the express consent of the court. After the consent was given, a forester pointed the trees and marked them. Thus, livestock such as specialised grass-eaters like cattle, horse and sheep would have found grasses and herbs in the 'Wald' and 'forestis'. Other data also point towards open grassland being an integral part of the 'Wald' and 'forestis'. In the Middle Ages the 'forestis' concerned treeless areas like open water and raised bogs. Raised bogs were also called 'Wald' or 'Wold'. Peat was cut in the 'Wald' in the 'forestis' (see LIGTENDAG, 1995, pp. 39, 41, 57, 65, 74, 77, 88, 176).
The 'Wald' and the 'forestis' also delivered wood, meaning the material, because there grew trees and shrubs. What delivered the wood (the material) was also called the wood. The place in which it was collected was called: in German 'Holz', 'holt' and 'wood'; in Dutch 'holt' and 'hout' and in Anglo Saxon 'holt'. The 'holt' could be delivered by single trees or trees that grew close together, forming a 'grove' or 'bush', in German 'Bush' and in Dutch 'bos'. This meant trees grouped together and therefore it distinguished itself as a clear entity from its surroundings. Because of that performance it was called a 'bush'. Areas in the 'forestis' or 'Wald' where wood was gathered were named after what they provided for human use, namely the material 'wood' (as in English in: a wooden house). So, the 'forestis', the 'Wald' contained 'weide' (pasture) and 'holt' (wood). The thing that all the areas that were called 'Wald', 'wold' or 'forestis' had in common is that it was the uncultivated land outside the cultivated, whether it contained trees or not.
The word 'forestis' evolved in German to 'Forst', in French to 'forêt', in English to 'forest' and in Dutch to 'foreest', 'voorst' and 'vorst'. The 'forestis' was called in the common language in German 'Wald and 'Wold', in Dutch 'wold', 'wald' and 'woud' and in Anglo-Saxon 'weald' (BORCK, 1954; KASPERS, 1957, pp. 154-156, 166-188; TRIER, 1963, pp. 45). Nowadays these words mean vast areas of closed forest, but in those days they all meant implicitly pasture for livestock. The pitfall with the language is that we nowadays read texts dating from the Middle Ages with the modern meaning of those concepts in our heads, namely a closed-canopy forest. So we translate those texts onto a closed-canopy forest, while that is not what the people in the Middle Ages meant (see VERA, 2000, pp. 109-111). As EVANS (2002) says: 'We don't use language, language uses us'.
Cattle grazing and the regeneration of trees
People also got permission to cut firewood in the 'forestis'. This was called coppicing. The earliest regulations on cutting firewood date from the 13th century. They refer mostly to the cutting of thorns, hazel and holly (HAUSTRATH, 1898, p44; 1928; TUBBS, 1964; 1988,p. 154; HART, 1966, pp.29- 30, 46-47, 128, 180-181, 308; STREITZ, 1967, p. 52; FLOWER, 1977, pp. 27, 63, 73). When firewood was cut, a certain number of saplings and young trees, especially of 'fruitful' trees like oak and wild fruit, had to be spared for every unit of area. They were chosen and marked by a 'forestarius' or forester according to the 'ius forestis' (see KASPERS, 1957, pp. 166; TUBBS, 1964, pp. 96; HART, 1966,pp. 95; WARTENA, 1968; RACKHAM, 1975,pp. 27; FLOWER, 1977, pp. 26; HAUSRATH, 1982, pp. 28-29; BUIS, 1985, pp. 304-305). These saplings and young trees had to grow up in order to provide the mast for pannaging pigs.
The place where the firewood was cut was called 'vorholt' and 'vorholtz' in German, 'voorhout' in Dutch (meaning the wood in front of the trees), 'underwood', 'brushwood' and 'shrubbery' in English, 'hage' in Anglo Saxon and 'petit taille et bordure' in French (VERA, 2000, pp. 139). Virtually all these names as well as the regulations concerning the saving of the young trees can be read as descriptions of mantle and fringe vegetation. There young trees grow up between thorns and hazel and wood-pastures (VERA, 2000, p. 139; see also TRIER, 1952, pp. 97, 115, 116; see Figure 3). Such a landscape consists of a mosaic of grassland, scrub, solitary trees and groves grazed by large ungulates like cattle and horse in combination with deer.
The stools of the thorny shrubs like blackthorn and of hazel do sprout after being cut. The roaming cattle could browse this spring growth, as well as the spared young trees, because their thorny shrubs, acting as protecting cages, were removed as firewood. Therefore, this is why the young trees had to be protected against the animals. This is in my opinion the reason why the regulations from the 13th century up to the 18th century were issued. They tell that what has been cut had to be closed for grazing until the new sprouts on the stools reached beyond the reach of the animals (see VERA, 2000, pp. 132-144). Commonly the grazing of livestock was forbidden for 3 up to 6 years. The time that is connected with the sprouting of the stools makes clear why a period of this length was issued. The sprouts on the stool of blackthorn do not bear thorns until the end of the first growing season. After one growing season those sprouts as well as those of hazel reach up to 2 metres high. After some years they are so thick that the animals cannot bend them downwards anymore with their neck in order to browse the twigs and leaves. After three years they are surely out of danger of the animals. If it were for the regeneration of seedlings within the forest - as foresters and ecologists claim - a prohibition for at least 15 to 20 years would have been necessary. This is the time needed for the stems to grow so thick that they can withstand the animals (see COTTA, 1865, pp. 84-85; TURBANG, 1954; FLOWER, 1977, pp. 198; MAYER, 1992, pp. 198; VERA 2000, p. 165-168). So the regulation of cattle grazing in woods was not to protect seedlings in closed forests, but to protect the vegetative regeneration of scrub and the seedlings and saplings of trees which grew up in the mantle and fringe vegetation of park-like grazed landscapes. None of these regulations on grazing livestock ever was aimed at regulating of livestock in general. They clearly state that the coppicing should be organised in such a way that it obstructed the rights to graze livestock as little as possible (see ENDRES, 1888, p. 91; HAUSRATH, 1898, p. 101; VANSELOW, 1926, p. 24, 145; HESMER and SCHROEDER, 1963, p. 152; MANTEL, 1980, p. 194, 195, 214; VERA, 2000, p. 136).
As mentioned above the regulations concerning the grazing of livestock corresponds with the regeneration of trees in wood-pastures protected by thorny shrubs. Thorny shrub and hazel mark, in wood-pastures, the transition of the grassland to groves, the so-called mantle and fringe vegetation. Trees do come up there in the thorny scrub. Young trees, particularly oaks, grow on the periphery of the scrub. This mantle and fringe vegetation advances into the grassland in a rate that is equal to the rate the outer edge of the scrub. The fringe extends into the grassland especially by blackthorn advancing into grassland by underground rootstocks (see WATT, 1924; BURRICHTER et al., 1980; POTT and HOPPE, 1991). The trees grow up in the thorny scrub and join the grove that is surrounded by the mantle and fringe vegetation, while this vegetation itself moves forward into the open grassland.
When firewood was cut, not all the young trees were spared, because the 'forestarii' did not mark every young tree. The reason they did so was that trees growing at some distance from each other form big crowns, flower and fruit more profusely and therefore produce more mast (acorns, pears, apples and cherries) for the pigs. So, some thinning among the young trees took place (see VERA, 2000, pp. 157-158). Therefore, beside the stools of shrubs also stools of trees came into existence that sprouted and ultimately formed part of the coppice. From these stools trees could be grown afterwards by sparing only one sprout on the stool. In this way during the Middle Ages out of scrub, and mantle, and fringe vegetation coppices with standards evolved (see Figure 5). The standards were the trees spared under the 'forest law'. Therefore they belonged to the lord. At first the standards were especially fruitful trees like oak, wild apple (Malus sylvestris), wild pear (Pyrus pyraster) and wild cherry (Prunus avium). These standards had to grow upwards in order to provide mast for the pigs. The trees were also used for timber. Later on also other tree species like, for instance, hornbeam became standards (see VERA, 2000, pp. 157-159).
The jay and the oak
A remarkable phenomenon in wood-pastures is the performance of oak in numbers as well as in shape. It is often stated that this is a consequence of human interference because the oak meant so much for people. There is however an explanation of how this may have happened in a natural way, namely because of the activity of an indigenous bird species, the jay (Garrulus glandarius). This species collects and plants acorns. Thereby it has a special preference for a transitional area of short to long vegetation. This is the transition between short and long grass, short and long herbs, grasses and herbs and the outer edge of hedges, in for instance the mantle and fringe vegetation bordering a grove. The jay also prefers the base of the stem of a hawthorn or juniper (CHETTLEBURGH, 1952; BOSSEMA, 1979, pp. 35, 45-47, 51, 57, 59). The clonal growth of blackthorn results in an ever-spreading group of trees, forming a grove. Because hawthorn lacks vegetative reproduction these shrubs will mostly protect a solitary oak that will ultimately result in an open grown tree. A jay may plant a single acorn at the base of a stem of juniper, resulting in an open grown oak as well. Juniper may also form layering branches in-between. Different jays may plant several acorns. This results in small groups of oaks spread in the open vegetation. This may also be the case with bramble.

FIGURE 5. Coppice with standards, central France. The standards are mainly oak, the shrub layer mainly hazel. Only a limited number of standards are preserved, as the coppice would otherwise decline significantly because of the shade cast by the trees. Photograph: F.W.M. Vera.
The crowns of the trees in a grove form a closed canopy. Because of the shade cast by the canopy, the scrub disappears. Within the groves no regeneration of trees takes place because of the shade cast by the canopy and the effects of the presence of large ungulates. If a gap is formed - most probably in the centre of the grove where the oldest trees are - grasses will establish there and attract large herbivores. Their grazing and trampling will prevent any tree seedling from coming up there. So they prevent the regeneration of trees in gaps of the canopy. As more trees die or are windblown, the surface of grassland increases. This process may also be initiated or hastened by fungi (GREEN, 1992; DOBSON and CRAWLY, 1994). In this way, ultimately the grove degrades from the centre onwards into grassland, as is well known and described as retrogressive succession. This process of degradation of the centre of the grove is well known, for instance from the wood-pastures of the New Forest, resulting in a call for fencing off the groves for large ungulates (see PETERKEN, 1996, p. 359; GORIUP, 1999; MOUNTFORD et al., 1999). In the long run, light-demanding thorny shrubs will establish in the grassland, protecting young trees against the large herbivores.
From the regulations concerning the grazing of cattle and cutting firewood it becomes clear that a 'forestis' or 'Wald' in written sources from the Middle Ages onwards was not a closed forest. It was the uncultivated wilderness consisting of grassland, trees and shrubs (inter alia light-demanding trees like oak, wild pear, wild cherry and wild apple and light-demanding shrubs like hawthorn and blackthorn and hazel) and even lakes and rivers. It was a park like landscape like a wood-pasture consisting of grassland, scrub trees and groves. The trees regenerated in thorny scrub. The jay played a major role in the establishment of oak in this landscape.
The oldest reference to this landscape comes from the Roman Tacitus in a description from Germania dating from AD 98. Germania was the territory on the other side of the river Rhine, which formed the border of the Roman Empire. He wrote: 'Terra, etsi aliquanto specie differt, in universum tamen aut silvis horrida aut paludibus foeda'. It means, 'The land, even though it is quite diverse, is generally either thorny in groves (ablatives limitationes) or swampy in marshes' (A.J. VAN WOLFEREN, DOORN, 1998, personal communication). Freely translated, it means: 'The land looks very different in many places, but in general, it is covered with thorny (bristling) woods (either thorny trees or thorny groves) and unhealthy marshes' (see VERA, 2000, pp. 119-120).
From coppice with standards to high forest with natural regeneration
In the 18th century the demand for firewood changed. Instead of bundles of faggots people wanted the firewood to be delivered in blocks. Therefore coppice rotation was extended from up to 10 years in the Middle Ages towards 30, 50 and even 80 years in the 18th century (VANSELOW, 1926, pp. 153; SCHUBART, 1966, pp. 98-99, 108, 126-127; MANTEL, 1990, pp. 366). As the rotation times extended the coppice with standards changed from brushwood with standards successively into pole forest with standard and eventually into a high wood, the woods we know today as timber wood.
In contrast to when stools are cut in short rotations, stools cut after 80 years do not sprout again. To obtain a new generation of trees, young trees had to be planted. Especially beech was favoured, because it was suitable for firewood in households and it produced the best charcoal for industry. In the 18th century the demand for charcoal by industry went up strongly, because of the industrial revolution. Beech usually flowers from the age of 20 to 30 years. So, with a rotation period longer than this beech flowered and formed seed. Seeds fell from the trees developed and into saplings. If the standing trees were gradually cut, the seedlings got more and more light. They could grow upwards successfully and form the new generation of trees to replace the harvested one. This is what was found out, and applied to the lowlands of Central and Western Europe in the German region Hessen in the first half of the 18th century. It formed the basis for the 'modem' forestry techniques today known as shelterwood cutting and selective cutting (see BUHLER, 1922, pp. 306, 324, 331, 353; SCHUBART, 1966, pp. 10}; MANTEL, 1990, pp. 361-362). These techniques were called 'natural' regeneration. They were opposite to artificial regeneration (see COTTA, 1865, pp. 2; VANSELOW, 1949, pp. 17; DENGLER, 1990, pg. 47). The natural aspect is, however, not that the technique was analogous to the regeneration in the natural situation. It was that the seedlings forming the new generation of trees emerge from seed that has fallen spontaneously from the standing trees. This was the opposite to sowing and planting, both of which were called artificial regeneration. Ploughing the ground in order to get a good germination bed for the seed and removing undesirable species of trees, shrubs, herbs and grasses were all part of the natural regeneration. Calling this technique 'natural' regeneration is therefore misleading.
The technique of 'natural' regeneration was first developed with the shade tolerant beech and very successful, because beech seedlings thrive well under the shelter of the old trees. They are shade-tolerant. Later on the technique was applied to the light-demanding oak as well. However during three-quarters of a century all efforts to regenerate oak 'naturally' failed. By trial and error one found out that oak needed much more daylight than beech. Therefore the seedlings had to be in full daylight much earlier than those of beech, because otherwise they would perish. In the case of beech it took about 40 years to have all the old trees successively removed and replaced by a new generation, while this had to take place within 10 years with oak. After that time much human assistance was still necessary to get the seedlings to successfully grow up. They had to be protected against shade-tolerant tree species, such as beech, lime and elm. Without this human interference the 'natural' regeneration of oak was doomed to fail, because the oaks will be ousted by the shade-tolerant species (VANSELOW, 1926, pp. 63, 87-88; KRAHL-URBAN, 1959, pp. 146; DENGLER, 1990, pp. 294). This empirical evidence from forestry shows that oak cannot regenerate spontaneously in a closed-canopy forest if shade-tolerant species are present.
With the application of this technique in the 'forestis' and the 'Wald' in the 18th and 19th century the grazing became a problem for the regeneration of the trees in a way we are now familiar with. The reason is that within the forest the young trees cannot be protected by thorny species, because these cannot thrive under the conditions of shade (see VERA, 2000, pp. 168-178). Therefore saplings and young trees were not protected against the livestock. Foresters propagated the separation of pasture for livestock and the production of wood. This became possible after the development of the so-called New Agriculture propagated by the fysiocrats. This development involved the introduction of the potato and fertilising via the cultivation of clover. In almost all of Europe this led in the course of the 18th and the 19th century to an agricultural reform (HOBE, 1805, p. 113; GROSSMANN, 1927, p. 29; BUIS, 1985, pp. 389, 520-521, 590; VERA, 2000, pp. 170-176). The 'modern' agriculture eventually led to the abolition of the commons in general and the abolition of grazing of the commoners in the 'forestis' or 'Wald' in particular all over Central and Western Europe. Wood-pastures and coppices with standards were changed into high forest. This type of forest is now considered to be the point of reference for the natural vegetation in the lowlands of Western and Central Europe. The way of regeneration of trees in the high forest became the baseline for the effect of large ungulates on the regeneration of trees. It is because of this that the grazing of large ungulates is in general considered to be harmful for the survival of trees.
In conclusion, foresters and forest and plant ecologists in the 19th century and the first half of the 20th century based their theory of the closed-canopy forest being the 'uncultivated wilderness' and the role of the large ungulates being detrimental for the regeneration of trees on an interpretation of these sources. In my opinion this is not correct. The same goes for the palynologists who on their turn build further on this interpretation with their interpretation of the pollen diagrams of the prehistoric vegetation. A final test of the theory of the closed-canopy forest being the primaeval vegetation can be done by looking at the results of the spontaneous development of the vegetation in the forest reserves.
The spontaneous development of forests
It follows that if the primaeval vegetation was a closed-canopy forest, all species of trees and shrubs which pollen studies have shown to be present in prehistoric times up to the introduction of agriculture will survive in spontaneously-developing closed-canopy forests. They have to regenerate there spontaneously when there are gaps in the canopy. This is supposed to happen in forest reserves, like La Tillaie and Le Gros-Fouteau in the forêt de Fontainebleau in France, Hassbruch, Neuenburger Urwald and Rohrberg in Germany, Dalby Soderskog in Sweden, the National Park Bialowieza in the Forest of Bialowieza in Poland and Suserup Skov and Draved Skov in Denmark. In these reserves there is a progressive replacement of the light demanding pedunculate oak (Quercus robur), sessile oak (Q. petraea) and hazel (Corylus avellana) by shade-tolerant tree species like beech (Fagus sylvatica), ash (Fraxinus excelsior), elm (Ulmus glabra and U. laevis), lime (Tilia platyphyllos and T. cordata) and hornbeam (Carpinus betulus) (see Figures 4 and 6). It is proved that the light-demanding oak is not able to regenerate either in small or large gaps nor in windblown areas of more than a hectare created by storms of outstanding intensity. Such storms release the advanced regeneration of shade-tolerant species, so that oak does not stand a chance against them (PIGOTT, 1975; DERKMAN and KOOP, 1977; LÖDL et al. 1977; MALMER et al., 1978; KOOP, 1981; 1989; AABY, 1983; LEMEE, 1985; EMBORG et al., 1996; PELTIER et al., 1997; PONTAILLER et al., 1997; HOUTZAGERS et al., 2000; VERA, 2000, pp. 189-286).
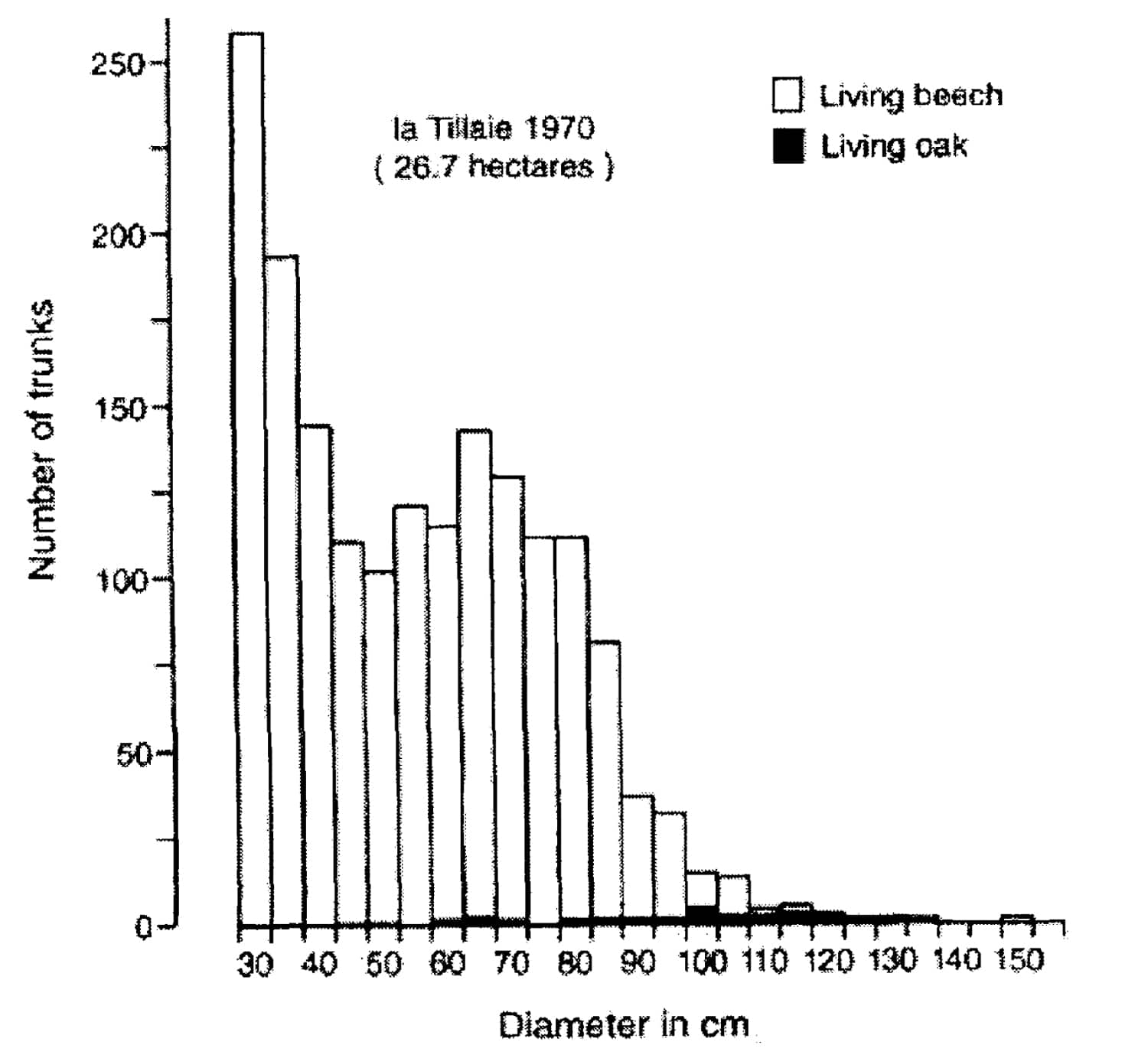
FIGURE 6. Distribution of sessile oak and beech in diameter categories of 5 cm in the whole of the reserve of La Tillaie (redrawn from LEMEE, 1978, p. 86).
As a shrub layer hazel does not thrive well in closed-canopy forests. If the closure of the canopy is more than 30 per cent, it hardly flourishes. In due time hazel even disappears in forest reserves where it initially is present because of the former history of those forests either as wood pasture or as coppice with standards (see inter alia NITZSCHKE, 1932; MALMER et al., 1978; HYTTEBORN, 1986; PETERKEN and JONES, 1987). For instance in the reserve Dalby Soderskog in Sweden the cover with hazel halved in 50 years time, after grazing ceased in this former wood-pasture and the forest became, because of that, closed (MALMER et al., 1978). This spontaneous development is not in agreement with pollen diagrams from Central and Western Europe in general and some of these reserves in particular (see for instance DABROWSKI, 1959 in. VERA, 2000, pp. 248-249; MITCHELL, 1998; MITCHELL and COLE, 1998). They show that both oak and hazel were very well represented in the primaeval vegetation. This goes in particular for the so-called Atlanticum, the period in North Western Europe, ranging from 9000 BP (Before Present with the reference year for Present of 1950) to 5000 BP. This is considered to be the period when the primaeval forest was optimally developed and no interference by man in the framework of agriculture was present yet. Oak and hazel thrived for many thousands of years in the presence of the shade tolerant species that nowadays oust oak and hazel in the forest reserves (see inter alia FIRBAS, 1934; 1935; 1949, p. 1; IVERSEN, 1941; 1960; 1973; GODWIN, 1975; VAN GEEL et al., 1981; HUNTLEY, 1986; 1988; HUNTLEY and BIRKS, 1983; DELCOURT and DELCOURT, 1987; BENNETT, 1988a,b,c; BERGLUND, 1991; BRADSHAW, 1993; HANNON et al., 2000; VERA, 2000, pp. 189-286).
All oaks present in these forest reserves date from the period when either wild or domestic cattle grazed in the forest or it was exploited as coppice with standards. The presence of oak in closed canopy forest reserves is in my opinion a result of the history of these reserves. Nowadays these areas accommodate a mixture of two systems. The first system is the wood-pasture where grazing large ungulates had a great influence. From this period oak in the presence of shade-tolerant species dates back to an earlier era. The second system is a closed-canopy forest where the influence of large grazing ungulates is excluded or minimised. In this system only the shade-tolerant tree species do regenerate and oak fails to do so. The combination results nowadays in the presence of old oaks with big shade-tolerant species like lime, elm and only new generations of shade-tolerant trees (see Figure 6). The combination of tree species in the present-day forest reserves therefore does not reflect the succession within a closed-canopy forest. Therefore in the forest reserves one does not see what one thinks one sees, namely a closed-canopy forest where oak is and will be present in the future.
As mentioned earlier, the light-demanding oak and hazel, together with the other shade-tolerant tree species mentioned, do regenerate very well in wood pastures, park-like landscapes where large typical grazers like cattle and horse, graze the vegetation. In this landscape the trees regenerate outside the forest in the mantle and fringe vegetation bordering the grove, forming the transition between grassland and grove. Inside the grove there is no regeneration because of the shade cast by the canopy and the grazing and trampling by the large ungulates present in the wood-pastures.
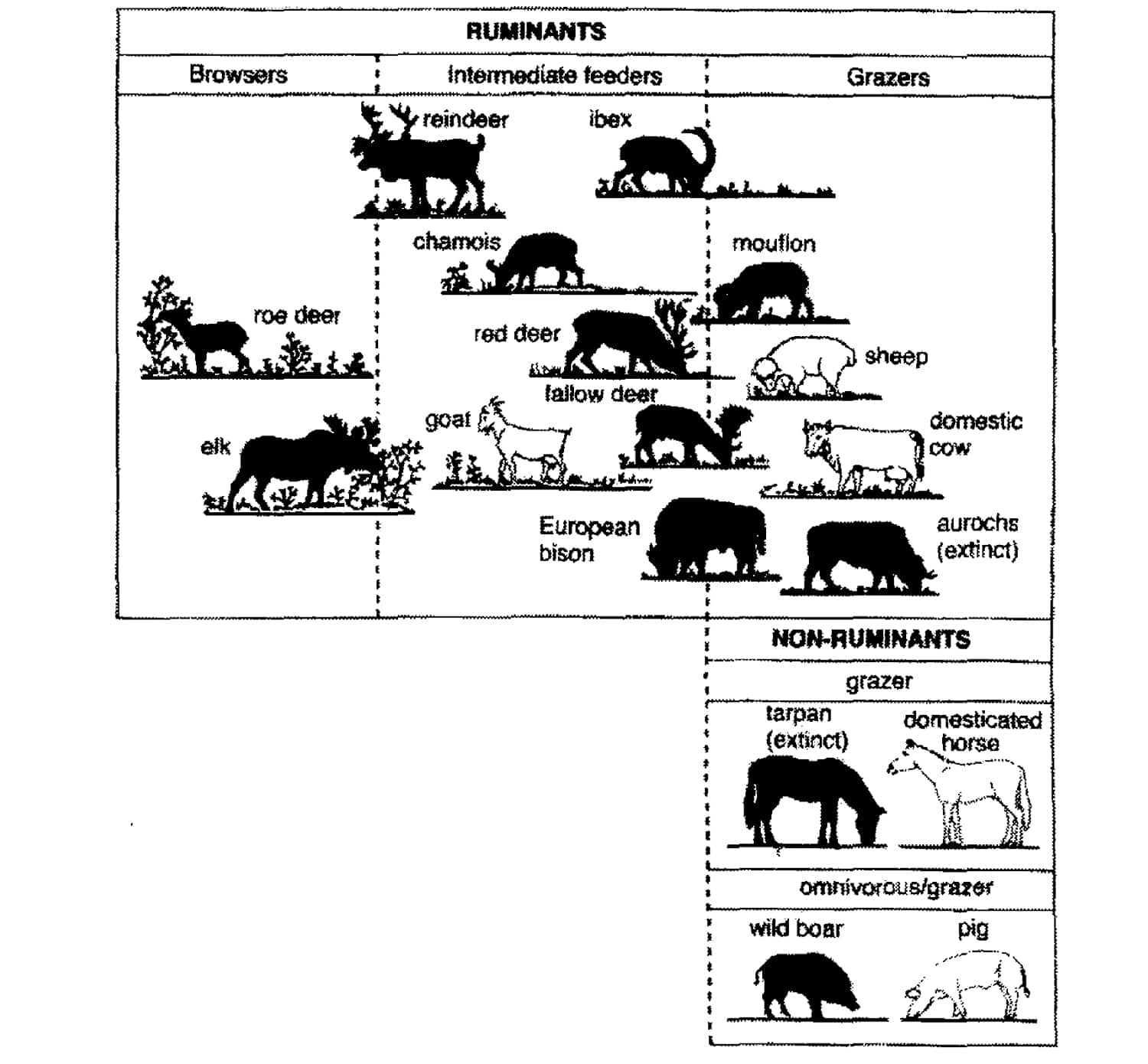
FIGURE 7. The different species of large ungulates indigenous in Europe, as well as the omnivorous wild boar, classified according to their feeding strategy. The domesticated species are shown in white. The indigenous species of the lowlands of central and western Europe include the aurochs, tarpan, European bison, red deer, moose, roe deer and wild boar (redrawn from HOFMANN, 1973; 1976; 1985; VAN DE VEEN and VANWIEREN, 1980).
It can be argued however, that wood-pastures are man-made systems, because the main actors in the processes steering the system are domesticated animals introduced by man. As far as their feeding habits are concerned (see HOFMANN, 1973, 1976, 1985), there is nevertheless no difference in principle between these domestic ungulates in the wood-pastures and the undomesticated wild fauna (see Figure 7). Cattle and domestic horse are descendants of respectively aurochs and tarpan, species that were part of the wild fauna and have the same feeding strategy. In former wood pastures livestock like cattle lived more or less like wild fauna, that is roaming freely (VERA, 2000, pp. 348-349). According to the old regulations it was forbidden to import or export fodder or animals from one common to another (ENDRES, 1888, pp. 13, 52; SLOET, 1911, pp. 118; BUIS, 1985, pp. 81). Therefore, the density of the domestic animals in wood pastures cannot have been beyond the carrying capacity of the wilderness, as must have been the case with the wild ungulates. Both the number of wild herbivores and that of livestock were regulated by the food supply. For the vegetation, this means that the presence and densities of livestock that made the establishment of trees and shrubs possible in wood pastures and can be seen as a modem analogy of this process in the past in the presence of the wild ungulates. The wild large ungulates must therefore have had densities that made the regeneration of oak and hazel possible, because otherwise the continued presence of oak and hazel in the primaeval vegetation cannot be explained (VERA, 2000, p. 349-350). These densities much have been reached in the presence of and the predation by large predators as wolf (Canis lupus), lynx (Lynx lynx) and brown bear (Ursus arctos) that were present in these times.
So, in conclusion, domestic livestock, which lived together with wild ungulates like deer and wild boar in wood pastures, can be considered as a modem analogy of the wild fauna including the wild progenitors of the domesticated species in the primaeval vegetation. The related vegetation - the wood pasture - has therefore to be considered as the closest modem analogy of the original vegetation. This primaeval park-like landscape must have been characterised by a high diversity of species of plants and animals, as is in wood-pastures nowadays.
What about the low percentage of arboreal pollen in pollen-diagrams?
If the primaeval vegetation was a park-like landscape, it must be responsible for pollen-diagrams with up to 90 per cent of tree pollen, the percentage that according to the current theory originates from a closed forest. After all, pollen diagrams constitute the factual information. If the primaeval vegetation was a park-like landscape there must be an explanation for the low percentage of herbs and grasses in these pollen diagrams. The following facts may explain the phenomenon.
The first is that in a park-like landscape such as wood pasture, thickets of blackthorn, hawthorn and hazel act as barriers to pollen of grasses and herbs. They prevent the horizontal movement of pollen from grasses and herbs by wind to raised bogs further on, where pollen samples have been taken. What contributes further to the prevention of the horizontal movement of pollen of grasses and herbs is that those thickets are arranged in a mosaic (see Figure 2). So, pollen that passes the first hurdle ends up in the second or third.
The second is that the large herbivores that graze the grass in park-like landscapes, at least partially prevent the grass from flowering and therefore from producing pollen. This means that the higher the densities of large grazing ungulates are, the lower the production of grass pollen per unit area will be. A low percentage of grass pollen in pollen diagrams may therefore be an indication of a high density of large grazing ungulates. As a consequence a rise of grass pollen in pollen diagrams may not mean an increase of the surface of grassland, but may be the result of a decrease in the number of grazing ungulates by disease or starvation.
The third is that in this park-like landscape, hazel in mantle vegetation and trees reaches high into the air and flower abundantly, producing much pollen. These can be picked up by air currents and, contrary to the pollen of grasses and herbs, transported over tens of kilometres to raised bogs from which pollen diagrams are derived.
All these factors separately may not explain why pollen of grasses and herbs are found in very low percentages in pollen diagrams of the primaeval vegetation compared with those of trees if the primaeval vegetation was a wood-pasture-like landscape. But they may do so by their accumulated effect. So, the combined effect of the first and the second fact can contribute to a low percentage of pollen of grasses and herbs, while the third fact combined with the combination of the other two will contribute to an overrepresentation of tree pollen and pollen of the shrub hazel compared to the pollen of grasses an herbs (see VERA, 2000, pp. 85-95). However, this theory should be tested. This can be done by modem pollen analyses of wood-pastures. There are a few modem pollen samples taken in such landscapes and some models made for areas with open spaces in a forest. They show that the percentage of non arboreal pollen (NAP) is a unreliable measure for the openness of the landscape in a sense that very open areas give spectra of pollen that are normally interpreted as being descended from closed-canopy forests (see BROSTROM et al., 1998; GAILLARD et al., 1998; SUGITA et al., 1999).
Synthesis
The development of the primaeval vegetation may be summarised as follows: In grazed park-like landscapes oaks and other tree species grow up in scrub or in the mantle and fringe vegetation of groves emerging in those grasslands, forming groves with a closed canopy. The grove advances into the grasslands at the speed of blackthorn advancing into grassland by underground rootstocks. Solitary trees will come up together with solitary hawthorns. Oak advances in such a landscape because jays plant the acorns at great distances from the fruiting oaks, right on the edge of shrubs. Seeds of other species are spread by the wind. The seedlings emerging from those can only survive if they come up in the direct vicinity of protective shrubs. On poorer soils trees can grow up amongst shrubs like juniper and bramble. Within the groves no regeneration of trees takes place because of the shade cast by the canopy and the effects of the presence of the large ungulates. Therefore oaks which are part of the grove will not be ousted by shade tolerant species. Concerning the regeneration of trees within the grove there is situation of stand-still. If a gap is formed, grasses will establish there as well as young trees. Light-demanding shrub species will not be able to establish themselves because there is still too much shade. The grasses will attract the large grazing herbivores. Their concentrated grazing and trampling will prevent seedlings of (shade tolerant) trees from coming up there, because they are not protected by thorny shrubs. So they prevent the regeneration of trees in gaps of the canopy. As more trees die or are windblown, the surface of grassland increases. This is the process that is well known as the retrogressive succession on the influence of grazing by large ungulates in forests. Fungi will facilitate the process of opening up the canopy and the demise of the trees (GREEN, 1992; DOBSON and CRAWLEY, 1994). In this way, ultimately the grove degrades from the centre onwards into grassland, as is known and described as retrogressive succession. At the same time the front of the grove will advance into the grassland.
On this general process some modifications are possible. They are dependent on the shrub species that nurse the tree species. They, in their tum are dependent on the type of soil. Blackthorn will cause characteristic concave shaped groves, because the shrub reproduces from a nucleus in every direction. Hawthorn will give way to solitary trees which will develop to open-grown trees. If the hawthorn becomes surrounded by blackthorn the open-grown tree will be surrounded by forest-grown trees that emerge outside its range of the crown in the spreading blackthorn. On more acid, poor soils bramble, juniper or even old shrubs of heather may act as nurses to trees (if the heather is grazed by cattle and not by sheep!). In these situations solitary trees or a few trees together will grow up in a further open space. Given these modifications the answer is still hidden as to the question of what natural circumstances are responsible for the features now observed in veteran oaks in the parks and wood-pastures in the UK. A theory from my side is that in a park-like landscape not all trees will die at the same time, because some species are more long-living than others. Long-living species like oak may survive and become trees surrounded by open space. These circumstances may facilitate the development towards the compact stature that is known from the so-called ancient or veteran trees.
In the long run, light-demanding thorny shrubs will establish in the newly developed open grassland, protecting young trees against the large herbivores. In this way a new grove emerges from the grassland. As has been shown earlier the view that large ungulates like cattle prevent the regeneration of trees does not apply as a general hypothesis. They facilitate the establishment in open grassland and prevent the regeneration in closed canopy forest. In fact they induce a non-linear succession, namely: grassland thorny shrubs grove grassland thorny shrub grove etc. I called this theory the cyclical turnover of vegetation (VERA, 2000, pp. 376-378).
This process started after the ending of the Last Ice Age, when the steppe tundra from the Ice Age came under modem temperatures, because of a rapid change of the climate (see COOPE, 1994; DANSGAARD et al., 1989). From this open land dominated by grasses and herbs, the fauna of the Ice Age like mammoth (Mammuthus primigenius) moved away, while the fauna we nowadays know as the indigenous fauna of wild ungulates of the Holocene, including wild oxen, the aurochs and wild horse, the tarpan, moved in. So the whole cyclical process started in an (almost?) open landscape.
Conclusions
The conclusion is that on places where large ungulates including large grazers like aurochs and tarpan could roam, the primaeval vegetation was not a closed-canopy forest. It was a park-like landscape with a very high diversity of biotopes and therefore a very high diversity of wildlife. The species diversity traditionally attributed to pre-industrial agricultural land - and in particular in grassland - is therefore not the result of the introduction of agriculture. It is the result of natural processes in which large herbivores, especially grass-eaters like cattle and horse played an essential role. They induced a non-linear succession, which resulted in a park-like landscape with a very high diversity of species. Much of this diversity survived since prehistoric times, even after the disappearance of the original large herbivores in wood pastures, because livestock in combination with the surviving wild ungulates formed more or less a modem analogue of the original situation in which the wild fauna steered the succession. The landscape and the underlying processes were therefore preserved, as well as the wildlife that was connected with the natural park-like landscape. However, over centuries, farming practice and forestry have developed in a way that is increasingly different from natural processes. The species diversity that was naturally found in a single interrelated system with large ungulates gradually became fragmented and distributed in all sorts of different types of agricultural and forested land. An example of that fragmentation is the separation of pasture and wood - the first becoming the modem grassland for milk and beef production, the latter the high woods and high forests for the production of wood. This was to the detriment of scrub and mantle and fringe vegetation and the large wild ungulates as well. The latter were ousted by agriculture or diminished by hunting in forests to unnatural low densities in order to protect the so-called 'natural' regeneration within the high forests in order to safeguard the regeneration of trees.
In view of the aims of nature conservationists - and botanists in particular - to retain the natural heritage, it might be necessary to retain the natural processes, or to redevelop them. This may mean agricultural land either being abandoned or taken out of production. There the interdependence and the interactions between large herbivores and the vegetation will have to be restored. Cattle, horse, red deer, elk, European bison, roe deer and wild boar will have to be able to live there as wild animals again. This means that cattle and horses will have to be rehabilitated as species living in the wild. Without these ungulates living in the wild the survival of the natural diversity will be impossible on the long run. The best place to start this process is in open grassland or heather in the neighbourhood of seed sources.
References
AABY, B. (1983). Forest development, soil genesis and human activity illutrated by pollen and hypha analysis of two neighbouring podzols in Draved Forest, Denmark. Danmarks Geologiske Undersøgelse II Raekke, Nr. 114 (Geological Survey of Denmark, 11, Series, No. 114).
AARIS-S0RENSEN, K. (1980). Depauperation of the Mammalian Fauna of the Island of Zealand during the Atlantic period. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening, 142, 131-138.
AARIS-S0RENSEN, K., PETERSON STRAND, K. and TAUBER, H. (1990). Danish finds of Mammoth (Mammuthus primigenius (Blumenbach)), stratigraphical position, dating and evidence of Late Pleistocene environment. Danmarks Geologiske Undersøogelse, B 14, (Geological Survey of Denmark No. 14), 1- 44.
AUGUSTE, P. and M. PATOU-MATHIS, M. (1994). L'aurochs au paléolithique. In: L. Bailly, L. and Cohën, A-S. de. (eds.) Aurochs, le retour. Aurochs, vaches et autres bovins de la préhistoire à nos jours. Centre Jurassien du Patrimoine, Lons-le-Saunier, pp. 13-26.
BENNETT, K.D. (1988a). Holocene pollen stratigraphy of central East Anglia, England, and comparison of pollen zones across the British Isles. New Phytologist, 109, 237-253.
BENNETT, K.D. (1988b). Post-glacial vegetation history: ecological considerations. In: Huntley, B. and Webb III, T. (eds.). Vegetation History, Section IV: Smaller scale studies. Handbook of Vegetation Science, Vol. 7, pp. 699-724.
BENNETT, K.D. (1988c). A provisional map of forest types for the British Isles 5.000 years ago. Journal of Quaternary Science, 4, 141-144.
BERGLUND, B.E. (1991). Environment and society in selected areas. Introduction; The Kopinge area; the Bjaresjo area; the Krageholm area; the Romele area. In: Berglund, B.E. (ed.) The cultural landscape during 6.000 years in southern Sweden. The Ystad Project. Ecological Bulletins, Copenhagen 41, pp. 109-112, 167-174, 221-224, 247-249.
BERTSCH, K. (1929). Klima, Pflanzendecke und Besiedlung Mitteleuropas in vor- und frühgeschichtlicher Zeit nach dem Ergebnissen der pollenanalystischen Forschung. Berichte der Römisch-Germanische Kommission des Deutschen Archeologischen Instituts, 18, 1-67.
BERTSCH, K. (1932). Die Pflanzenreste der Pfahlbauten von Sipplingen und Langenrain im Bodensee. Badische Fundberichte, 2, 305-320.
BERTSCH, K. (1949). Geschichte des deutschen Waldes. Jena.
BORCK, K-H. (1954). Zur Bedeutung der Wörter Holz, Wald, Forst und Witu im Althochdeutschen. Festschrift für Jost Trier, Meisenheim, pp. 456-476.
BOSSEMA, J. (1979). Jays and Oaks: An Eco-Ethological Study of a Symbiosis. PhD. Thesis Rijksuniversiteit Groningen, Groningen. (Also published in Behaviour, 70, 1-117).
BRADSHAW, R. (1993). Forest response to Holocene climatic change: equilibrium or non-equilibrium. In: Chambers, F.M. (ed.) Climate Change and Human Impact on the Landscape. Chapman & Hall, London, pp. 57-65.
BROSTRÖM, A., GAILLARD, M.-J., IHNSE, M. and ODGAARD, B. (1998). Pollen landscape relationships in modem analogues of ancient cultural landscapes in southern Sweden - a first step towards quantification of vegetation openness in the past. Vegetation History and Archaeobotany, 1, 189-201.
BÜHLER, A. (1922). Der Waldbau nach wissenschaftlicher Forschung und praktischer Erfahrung. II Band. Eugen Ulmer, Stuttgart.
BUIS, J. (1985). Historia Forestis: Nederlandse bosgeschiedenis. Deel 1 en 2. H & S Uitgevers, Utrecht.
BUIS, J. (1993). Holland Houtland. Een geschiedenis van het Nederlandse bos. Prometheus, Amsterdam.
BURRICHTER, E. (1977). Vegetationsbereicherung und Vegetationsverarmung unter dem Einfluss des prähistorischen und historischen Menschen. Natur und Heimat, 37, 46-51.
BURRICHTER, E. POTT, R. RAUS, T. and R. WITTIG, R. (1980). Die Hudelandschaft "Borkener Paradies" im Emstal bei Meppen. Abhandlungen aus den Landesmuseum für Naturkunde zu Münster in Westfalen. Mtinster 42. Jahrgang, 4.
CHETTLEBURGH, M.R. (1952). Observations on the collection and burial of acorns by jays in Hinault Forest. British Birds 45, 359-64. Also further note (1955): 48, 183-4.
CLEMENTS, F.E. (1916). Plant succession. An analysis of the development of vegetation. Publication nr. 242. Carnegie Institution, Washington D.C.
COOPE, G.R. (1994). The response of insect faunas to glacial-interglacial climatic fluctuations. Philosophical Transactions of the Royal Society of London Series B, 344, 19-26.
COTTA, H. (1865). Anweisung zum Waldbau. (Neunte, neubearbeitete Auflage) Arnoldische Buchhandlung, Leipzig.
DABROWSKI, M.J. (1959). Late-glacial and Holocene History of Bialowiezá Primeforest. Part I., Bialowiezá National Park. Acta Societatis Botanicorum Poloniae, 28, 197-248.
DANSGAARD, W., WHITE, J.W.C. and JoHNSEN, S.J. (1989). The abrupt termination of the Younger Dryas climate event. Nature, 339, 532-534.
DEGERB0L, M. (1964). Some remarks on Late- and Post-glacial vertebrate fauna and its ecological relations in northern Europe. In: Macfadyen, A. and Newbould, P.J. (eds.) British Ecological Society Jubilee Symposium. London, 28-30 March 1963. Journal of Ecology 52, 71-85, Journal of Animal Ecology, 33.
DEGERB0L, M. and IVERSEN, J. (1945). The Bison in Denmark. Danmarks Geologiske Undersøgelse II. Raekke Nr. 73 (Geological Survey of Denmark No. 73).
DELCOURT, P.A. and DELCOURT, H.R. (1987). Late-Quatarnary dynamics of temperate forests: applications of palaeoecology to issues of global environmental change. Quaternary Science Reviews, 6, 129-146.
DE MONTE VERLOREN, J.P.H. and SPRUIT, J.E. (1982). Hoofdlijnen uit de ontwikkeling der rechterlijke organisatie in de Noordelijke Nederlanden tot de Bataafse omwenteling. 6th edn. Kluwer, Deventer.
DENGLER, A. (1935). Waldbau auf ökologischer Grundlage, 2th. edn. Berlin.
DENGLER, A. (1990). Waldbau auf ökologischer Grundlage, Zweiter band. Baumartenwahl, Bestandesbegründung und Bestandespflege. 6th. edn. by Rohrig, E. and Gussone, H.A. Verlag Paul Parey, Hamburg and Berlin.
DERKMAN, G.F.M. and KOOP, H.G.J.M. (1977). Structuur en verjonging van een oerbos. Praktijkverslag Natuurbehoud en Natuurbeheer. Landbouwhoge school, Wageningen, LH/NG, projectnr. P2, Wageningen.
DOBSON, A., CRAWLEY, M. (1994). Pathogens and the structure of plant communities. Trends in Ecology and Evolution, 9, 303-398.
ELLENBERG, H. (1986). Vegetation Mitteleuropas mit den Alpen in ökologischer Sicht. Vierte, verbesserte Auflage. Verlag Eugen Ulmer, Stuttgart.
ELLENBERG, H. (1988). Vegetation Ecology of Central Europe. 4th edn. Cambridge University Press, Cambridge.
EMBORG, J., CHRISTENSEN, M., HEILMAN-CLAUSEN, J. (1996). The Structure of Suserup Skov, a Near-Natural Temperate Deciduous Forest in Denmark. Forest and Landscape Research, 1, 311-333.
ENDRES, M. (1888). Die Waldbenutzung von 13. bis Ende des 18. Jahrhunderts. Ein Beitrag zur Geschichte der Forstpolitik. Verlag der H. Laupp'schen Buchhandlund, Tilbingen.
EVANS, P. (2002). A night of dark trees. Arboricultural Journal, 26(3), 249- 256.
FIRBAS, F. (1934). Über die Bestimmung der Walddichte und der Vegetation Waldloser Gebiete mit Hilfe der Pollenanalyse. Planta, 22, 109-146.
FIRBAS, F. (1935). Die Vegetationsentwicklung des Mitteleuropäischen Spätglacials. Bibliotheca Botanica, 112, 1-68.
FIRBAS, F. (1949). Spät- und nacheiszeitliche Waldgeschichte Mitteleuropas nördlich der Alpen. Erster Band: Allgemeine Waldgeschichte. Verlag von Gustav Fischer, Jena.
FLOWER, N. (1977). An Historical and Ecological Study of Inclosed and Uninclosed Woods in the New Forest, Hampshire. MSc thesis, King's College, University of London.
FORBES, A.C. (1902). On the regeneration and formation of woods from seed naturally of artificially sown. Transactions of the English Arboricultural Society, 5, 239-270.
GAILLARD, M.-J. BIRKS, H.J.B. IHSE, M. and RUNBERG, S. (1998). Pollen/landscape calibrations based on modern pollen assemblages from surface sediments samples and landscape mapping- a pilot study in south Sweden. In: Gaillard, M.-J. and Berglund, B.E. (eds.) Quantification of land surfaces cleared of forest during the Holocene-Modern pollen/vegetation/landscape relationships as an aid to the interpretation of pollen data. Paläoklima-forschung/Palaeoclimate Research, 1, 31-52.
GEEL, B. van, BOHNCKE, S.J.P. and DEE, H. (1980/1981). A palaeoecological study of an upper Lateglacial and Holocene sequence from "De Borchert", the Netherlands. Review of Palaeobotany and Palynology, 31, 367-348.
GODWIN, H. (1934,a). Pollen analysis. An outline of the problems and potentialities of the method. Part. I. Technique and interpretation. New Phytologist, 33, 278-305.
GODWIN, H. (1934,b). Pollen analysis. An outline of the problems and potentialities of the method. Part. II. General applications of pollen analysis. New Phytologist, 33, 325-358.
GODWIN, H. (1975). The History of the British Flora. 2th edn. Cambridge University Press, Cambridge.
GORIUP, P. (ed.) (1999). The New Forest Woodlands. A Management History. Forestry Commission, Pisces Publications for The Forestry Commission, Berkshire.
GREEN, T. (1992). The Forgotten Army- Woodland Fungi. British Wildlife, 2, 85-86.
GROSSMANN, H. (1927). Die Waldweide in der Schweiz. Promotionsarbeit, Zürich.
HANNON, G.E., BRADSHAW, R. and BMBORG, J. (2000). 6000 years of forest dynamics in Suserup Skov. A seminatural Danish woodland. Global Ecology and Biogeography, 9, 101-114.
HART, G.E. (1966). Royal Forest. A History of Dean's Woods as Producers of Timber. Clarendon Press, Oxford.
HAUSRATH, H. (1898). Forstgeschichte der rechtsrheinischen Theile des ehemaligen Bisthums Speyer. Julius Springer, Berlin.
HAUSRATH, H. (1982). Geschichte des deutschen Waldbaus. Von seinen Anfängen bis 1850. Hochschulverlag Freiburg (Breisgau).
HESMER, H. (1958). Wald- und Forstwirtschaft in Nordrhein-Westfalen., Hannover.
HESMER, H. and SCHROEDER, F-G. (1963). Waldzusammensetzung und Waldbehandlung im Niedersächsischen Tiefland westlich der Weser und in der Münsterschen Bucht bis zum Ende des 18 Jahrhunderts. Forstgeschichtlicher Beitrag zur Klärung der natürlichen Holzartenzusam-mensetzung und ihrer künstlichen Veränderungen bis in die frühe Waldbauzeit. Decheniana, Beiheft 11, pp. 1-304.
HOBE, J.H. von (1805). Freymüthige Gedanken über verschiedene Fehler bey dem Forsthaushalt, insbesondere über die Viehude in den Holzungen, deren Abstellung und Einschränkung. Thal-Ehrenbreitstein, in der Gehraschen Hofbuchhandlund.
HOFMANN, R.R. (1973). The Ruminant Stomach: Stomach Structure and Feeding Habits of East African Game Ruminants. East African Literature Bureau, Nairobi, Kenya.
HOFMANN, R.R. (1976). Zur adaptiven Differenzierung der Wiederkäuer: Untersuchungsergebnisse auf der Basis der vergleichenden funktionellen Anatomie des Verdauungstrakts. Praktische Tierärtzt, 57, 351-358.
HOFMANN, R.R. (1985). Digestive Physiology of the Deer. Their Morphophysiological Specialisation and Adaptation. The Royal Society of New Zealand Bulletin, 22, 393-407.
HOUTZAGERS, M. NEUTEL, W. ROSSEEL, A. and SWART, B. (2000). Fontainebleau (re)visited. Effect van storm in bosreservaat in Fontainebleau. Nederlands Bosbouw Tijdschrift, 72, 228-233.
HUNTLEY, B. and BIRKS, H.J.B. (1983). A atlas of past and present pollen maps of Europe: 0-13.000 years ago. Cambridge University Press, Cambridge.
HUNTLEY, B. (1986). European Post-glacial Vegetation History: a New Perspective. In: Ouellet, H. (ed.) Acta XIX Congressus Internationalis Ornithologici (Vol. I). Natural Museum of Natural Sciences, University of Ottowa Press, pp. 1061-1077.
HUNTLEY, B. (1988). Europe. Vegetation History. In: Huntley, B. and Webb III, T. (eds.) Vegetation History. Section III: Glacial and holocene vegetation history -20 ky to present. Handbook of Vegetation Science, Vol. 7. Kluwer, Dordrecht, pp. 341-382.
HYTTEBORN, H. (1986). Methods of forest dynamics research. In: Fanta, I. (ed.) Forest dynamics research in Western and Central Europe. Pudoc, Wageningen,pp. 17-31.
IVERSEN, J. (1941). Land Occupation in Denmark's Stone Age. A Pollen Analytical Study of the Influence of Farmer Culture on the Vegetational Development. Danmarks Geologiske Undersøgelse, II. Raekke nr. 66. (Geological Survey of Denmark No. 66).
IVERSEN, J. (1960). Problems of the Early Post-Glacial Forest Development in Denmark. Danmarks Geologiske Undersøgelse, IV. Raekke Bd. 4, nr 3 (Geological Survey of Denmark. IV Series Vol. 4 No. 3).
IVERSEN, J. (1973). The Development of Denmark's Nature since the Last Glacial, Danmarks Geologiske Undersøgelse, V. Raekke nr. 7-c (Geological Survey of Denmark. V. Series No. 7-c ).
JAHN, G. and RABEN, G. (1982). Über den Einfluss der Bewirtschaftung auf Struktur und Dynamik der Wälder. In: H. Dierschke, H. (ed.) Struktur und Dynamik von Wäldern Berichte der Intemationale Symposium der Intemationalen Verein fiir Vegetationskunde, J. Cramer, Valduz., 717-734.
KASPERS, H. (1957). Comitatus nemoris. Die Waldgrafschaft zwischen Maas und Rhein. Beiträge zur Geschichte des Dürener Landes, Band 7, Düren und Aachen.
KOOP, H. (1981). Vegetatiestructuur en dynamiek van twee natuurlijke bossen: het Neuenburger en Hasbrucher Urwald. Pudoc Centrum voor Landbouwpublicaties en Landbouwdocumentatie, Wageningen.
KOOP, H. (1989). Forest Dynamics. Silvi-Star: A Comprehensive Monitoring System. Springer Verlag, Berlin.
KRAHL-URBAN, J. (1959). Die Eichen. Forstliche Monographie der Traubeneiche und der Stieleiche. Paul Parey, Berlin.
LEIBUNDGUT, H. (1959). Über Zweck und Methodik der Struktur und Zuwachsanalyse von Urwäldern. Schweizerische Zeitschrift für Forstwesen, 110, 111-124.
LEIBUNDGUT, H. (1978). Über die Dynamik europläscher Urwälder. Allgemeine Forstzeitschrift, 33, 686--690.
LEMÉE, G. (1985). Role des arbres intolérants à l'ombrage dans la dynamique d'une hêtraie naturelle (forêt de Fontainebleau). Oecologia Plantarum, 6, 3-20.
LEMÉE, G. (1987). Les populations de chênes (Quercus petraea Liebl.) des réserves biologiques de La Tillaie et du Gros Fouteau en forêt de Fontainebleau: structure, demographie et évolution. Revue d'Ecologie, 42, 329-355.
LIGTENDAG, W.A. (1995). De Wolden en het water. De landschaps- en waterstaatsontwikkeling in het Lege land ten oosten van de stad Groningen vanaf de voile Middeleeuwen tot ea. 1870. Regio en Landschapsstudies nr.2. Stichting Historisch Onderzoek en Beleid. REGIO-PRoject Uitgevers, Groningen.
LÖDL, J., MAYER, H. and P1TTERLE, A. (1977). Das Eichen-Naturschutzgebiet Rohrberg im Hochspessart. Forstwissenschaftliches Centralblatt, 96, 294- 312.
MALMER, N., LINDGREN, K. and PERSSON, S. (1978). Vegetational succession in a south-Swedish deciduous wood. Vegetatio, 36, 17-29.
MANTEL, K. (1980). Forstgeschichte des 16. Jahrhunderts unter dem Einfluft der Forstordnungen und Noe Meurers. Paul Parey, Hamburg und Berlin.
MANTEL, K. (1990). Wald und Forst in der Geschichte. M. und H. Schaper, Alfeld-Hannover.
MAYER, H. (1992). Waldbau auf soziologisch-okologischer Grundlage, 4., teilweise neu bearbeitete Auflage. Gustav Fischer, Stuttgart.
MEYER, K.A. (1931). Geschichtliches von den Eichen in der Schweiz. Mitteilungen der Schweizerischen Centralanstalt für das forstlichen Versuchswesen 16, 231-452.
MITCHELL, F.J.G. (1998). The investigation of long-term succession in temperate woodland using fine spatial resolution pollen analysis. In: Kirby, K.J. and WATKINS, C. (eds.) The ecological history of European Forests. CAB International Wallingford, United Kingdom, pp. 213-223.
MITCHELL, F.J.G. and COLE, E. (1998). Reconstruction of long-term successional dynamics of temperate woodland in Bialowieza Forest, Poland. Journal of Ecology, 86,1042-1059.
MOSS, C.E. (1910). The Fundamental Units of Vegetation: Historical Development of the Concepts of the Plant Association and the Plant Formation. New Phytologist, 9, 18-53.
MOUNTFORD, E.P., PETERKEN, G.F., EDWARDS, P.J., MANNERS, J.G. (1999). Long-term change in growth, mortality and regeneration of trees in Denny Wood, an old-growth wood-pasture in the New Forest (UK). Perspectives in Plant Ecology, Evolution and Systematics, 2, 223-272.
MULLER, F. and RENKEMA, E.H. (1995). Beknopt Latijns-Nederlands Woordenboek. 12th. edn. Wolters-Noordhoff, Groningen.
NITZSCHKE, H. (l 932). Der Neuenburger Uwrwald bei Bockhorn in Oldenburg. Vegetationsbilder 23(6/7). Gustav Fischer, Jena.
PEGLAR, S.M. (1993). The mid-Holocene Ulmus-decline at Diss Mere, Norfolk, UK: a year-by-year pollen stratigraphy from annual laminations. The Holocene, 3, 1-13.
PELTIER, A., TOEZET, M.-C., ARMENGAUD, C. and PONGE, J.-F. (1997). Establishment of Fagus sylvatica and Fraxinus exceslsior in an old-growth beech forest. Journal of Vegetation Science, 8, 13-20.
PETERKEN, G.F. (1996). Natural Woodland. Ecology and Conservation in Northern Temperate Regions. Cambridge University Press, Cambridge.
PETERKEN, G.F. and TUBBS, C.R. (1965). Woodland regeneration in the New Forest Hampshire, since 1650. Journal of Applied Ecology, 2, 159-170.
PETERKEN, G.F. and JONES, E.W. (1987). Forty years of change in Lady Park Wood: the old growth stands. Journal of Ecology, 75, 477-512.
PIETZARKA, U. and ROLOFF, A. (1993). Dynamische Waldrandgestaltung. Ein Modell zur Struckturverbesserung von Wald aussenriindern. Natur und Landschaft, 68, 555-560.
POGOTT, C.D. (1975). Natural regeneration of Tilia cordata in relation to forest-structure in the forest of Bialowieza, Poland. Philosophical Transactions of the Royal Society of London Series B, 270, 151-179.
PONTAILLER, J.-Y. FAILLE, A. and LEMÉE, G. (1997). Storms drive successional dynamics in natural forests: a case study in Fontainebleau forest (France). Forest Ecology and Management, 98, 1-15.
POST, L. VON (1916). Forest tree pollen in South Swedish Peat Bog Deposits. (Om skogstradspollen i sydvenska torfmosselager foljder (foredragsreferat). Geologiska Foereningen in Stockholm, Four handlingar, 38, 384-434. Translation by Margaret Bryan Davis and Knut Faegri with an introduction by Knut Faegri and Johs. Iversen. Pollen et Spores 1967, 9, 378-401. In: Real, L.A. and BROWN, J.H. (eds.) Foundations of Ecology. Classic Papers with commentaries The University of Chicago Press, Chicago, London, pp. 456-482.
POTT, R. and HÜPPE, J. (1991). Die Hudenlandschaften Nordwestdeutschlands. Westfälisches Museum für Naturkunde, Landschafsverband Westfalen-Lippe. Veröffentlichung der Arbeitsgemeinschaft fiir Biol.-ökol. Landesforschung, ABÖL, nr. 89, Münster.
PUTMAN, R.J., EDWARDS, P.J., MANN, J.C.E., HOW, R.C. and HILL, S.D. (1989). Vegetational and Faunal Changes in an Area of Heavily Grazed Woodland Following Relief of Grazing. Biological Conservation, 47, 13-22.
RACKHAM, 0. (1975). Hayley Wood. Its History and Ecology. Cambridgeshire and Isle of Ely Naturalists' Trust. Cambridge.
RACKHAM, 0. (1980). Ancient Woodland. Its history, vegetation and uses in England. Edward Arnold, London.
RODWELL, J.S. (ed.) (1991). British Plant Communities. Volume I. Woodlands and Scrub. Cambridge University Press, Cambridge.
SCHUBART, W. (1966). Die Entwicklung des Laubwaldes als Wirtschaftswald zwischen Elbe, Saale und Weser. Aus dem Walde. Mitteilungen aus der Niedersächsischen Landesforstverwaltung 14.
SLOET, J.J.S. BARON (1911). Gelderse markerechten. Oud-vaderlandse rechtsbronnen. Werken der Vereeniging tot Uitgaaf der Bronnen van het Oud Vaderlands Recht, Utrecht. Tweede reeks no. 12, Martinus Nijhoff, 's Gravenhage.
STREITZ, H. (1967). Bestockungswandel in Laubwaldgesellschaften des Rhein Main-Tieflandes und der Hessischen Rheinebene. Dissertation Forstlichen Fakultät der Georg-August-Universitat zu Göttingen in Hannover, Münden.
SUGITA, S., GAILLARD, M.-J. and BROSTÖM, A. (1999). Landscape openness and pollen records: a simulation approach. The Holocene, 9, 409-421.
TANSLEY, A.G. (ed.) (1911). Types of British Vegetation. Cambridge University Press, Cambridge.
TANSLEY, A.G. (1935). The Use and Abuse of Vegetational Concepts and Terms. Ecology 16, 284-307.
TANSLEY, A.G. (1953). The British Islands and their Vegetation. Vols 1 and 2, 3th edn. Cambridge University Press, Cambridge.
TILMAN, D. (1985). The resource-ratio hypothesis of plant succession. American Naturalist, 125, 827-852.
TRIER, J. (1952). Holz. Etymologien aus dem Niederwald. Münstersche Forschungen 6, Bohlau Verlag, Mtinster/Koln.
TSCHADEK, G. (1933). Die Waldentwicklung Mitteleuropas im Lichte der pollenanlytischen Ergebnisse und der urgeschichtlichen Forschung. Centralblatt für das gesamte Forstwesen, 59, 206-213.
TUBBS, C.R. (1964). Early Encoppicements in the New Forest. Forestry, 37, 95-105.
TURBANG, J. (1954). Contribution à l’étude de la régéneration naturelle du Chêne en Lorraine Beige. Bulletin Institut Agronomique et Stations de Recherches de Gembloux (Belgium), 22, 90-133.
VANSELOW, K. (1926). Die Waldbautechniek im Spessart. Eine historisch kritische Untersuchung ihrer Epochen. Verlag von Julius Springer, Berlin.
VANSELOW, K. (1949). Theorie und Praxis der naturlichen Verjungung im Wirtschaftswald. Neumann Verlag, Berlin.
VEEN, H.A., VAN DE and WIEZEN, S.A. VAN (1980). Van grote grazers, kieskeurige fynpwevens en opportunistische gelegenheid sureters; over het gebruk van groter herbivoren bij de ontoislekeling en duu z zame instandhouding van natuurwaarden. Rapport 80/11. Instituut voor Milieuvraagstukken, Amsterdam.
VEEN, P.A.F. VAN and Sns, N. VAN DER (1990 en 1991). Etymologisch woordenboek. De herkomst van onze woorden. Van Dale Lexicografie, Utrecht/Antwerpen.
VERA, F.W.M. (2000). Grazing Ecology and Forest History. CABI Publishing, Wallingford.
VRIES, J. de (1970). Etymologisch woordenboek. Waar komen onze woorden vandaan? Het Spectrum, Utrecht/Antwerpen.
WARTENA, R. (1968). Vier eeuwen bosbeheer in Gelderland 1400-1800. Tijdschrift Koninklijke Nederlandse Heidemij, 19, 33-40, 94-100, 182-191. WATT, A.S. (1919). On the causes of failure of natural regeneration in Britisch oakwoods. Journal of Ecology, 1, 173-203.
WATT, A.S. (1924). On the ecology of British beech woods with special reference to their regeneration. Part II. The Development and Structure of Beech Communities on the Sussex Downs. Journal of Ecology, 12, 145-204. WATT, A.S. (1947). Pattern and process in the plant community. Journal of Ecology, 35, 1-22.
Résumé
On accepte généralement la théorie que, sans l”intervention humaine, ce serait une forêt présentant un couvert fermé qui prédominerait en Europe centrale et occidentale aux endroits où Jes arbres peuvent pousser. La preuve apparente de l’existence passée d’une forêt au couvert fermé provient de plusieurs sources: Jes pâturages abandonnés retoumant à l’état de forêt; les études palynologiques; et l’analyse Iinguistique. Or, VERA (2000) montre que ces concepts sont erronés en plusieurs points-clés. Le chêne (Quercus species) et le noisetier (Corylus avellana) sont des essences exigeant de la lumière et leur présence dans les études palynologiques, ainsi que celle de nombreux ossements de bovins et de chevaux, indique en fait la présence de terrain decouvert. L’analyse linguistique montre aussi que les terms de “forêt” et de “bois” n’impliquaient pas uniquement la présence d’un couvert continu d’arbres, mais plutôt d’un sol non cultivé-à savoir ni labouré, ni fauché-avec ou sans arbres et arbustes-situé “a l’exterieur du village.” Ces nouvelles interprétations se combinent avec l’évidence du rôle joué par l’épine noire (Prunus spinosa) et avec le broutement de grands ongulés comme Jes bovins (sauvages) et chevaux (sauvages) en tant qu’aide à la régénération des broussailles (et de la futaie). L’article conclut que l’Europe n’a pas été entièrement couverte par une forêt présentant un couvert fermé aux endroits où les arbres sont en mesure de pousser. Aux endroits où il était possible aux bovins sauvages et aux chevaux sauvages de vivre en compagnie d’autres grands ongulés, cette dernière était à l’origine constituée de bois-patûrages dynamiques.